 对照组,根据是否有嗜烟史又将两组各分为嗜烟组和不嗜烟组。各组患者行支气管镜检查并行支气管肺泡灌洗,检测各组BALF中SP-D的表达。(2)培养人类Ⅱ型肺泡上皮细胞系,分为对照组和香烟烟雾提取物组,对照组:细胞在无血清的培养液中培养。香烟烟雾提取物组:在培养液中加入香烟烟雾提取物培养。12 h 后检测SP-D的表达,然后予地塞米松加入培养液中干预,72 h 后检测培养液中SP-D的水平。 结果 COPD组患者BALF中SP-D较对照组表达明显降低。COPD组中嗜烟组BALF中SP-D明显低于不嗜烟组。对照组中嗜烟组BALF中SP-D明显低于不嗜烟组。体外实验表明,香烟烟雾提取物干预组细胞培养液中SP-D的表达较对照组明显降低,而经地塞米松处理后香烟烟雾提取物干预组细胞培养液中SP-D表达明显增加,对照组经地塞米松处理前后SP-D表达没有明显变化。 结论 COPD患者BALF中SP-D的表达明显降低。嗜烟可明显下调BALF中SP-D的表达。香烟烟雾提取物干预的Ⅱ型肺泡上皮细胞培养液中SP-D的表达明显降低,激素对Ⅱ型肺泡上皮细胞SP-D的表达具有上调作用。
对照组,根据是否有嗜烟史又将两组各分为嗜烟组和不嗜烟组。各组患者行支气管镜检查并行支气管肺泡灌洗,检测各组BALF中SP-D的表达。(2)培养人类Ⅱ型肺泡上皮细胞系,分为对照组和香烟烟雾提取物组,对照组:细胞在无血清的培养液中培养。香烟烟雾提取物组:在培养液中加入香烟烟雾提取物培养。12 h 后检测SP-D的表达,然后予地塞米松加入培养液中干预,72 h 后检测培养液中SP-D的水平。 结果 COPD组患者BALF中SP-D较对照组表达明显降低。COPD组中嗜烟组BALF中SP-D明显低于不嗜烟组。对照组中嗜烟组BALF中SP-D明显低于不嗜烟组。体外实验表明,香烟烟雾提取物干预组细胞培养液中SP-D的表达较对照组明显降低,而经地塞米松处理后香烟烟雾提取物干预组细胞培养液中SP-D表达明显增加,对照组经地塞米松处理前后SP-D表达没有明显变化。 结论 COPD患者BALF中SP-D的表达明显降低。嗜烟可明显下调BALF中SP-D的表达。香烟烟雾提取物干预的Ⅱ型肺泡上皮细胞培养液中SP-D的表达明显降低,激素对Ⅱ型肺泡上皮细胞SP-D的表达具有上调作用。
[關键词] 肺表面活性物质D;慢性阻塞性肺疾病;香烟烟雾;糖皮质激素;支气管肺泡灌洗液
[中图分类号] R562.1+2 [文献标识码] A [文章编号] 1673-9701(2017)08-0001-04
[Abstract] Objective To investigate the expression of SP-D in BALF in COPD patients and smokers, and to study the effects of cigarette smoke on the expression of SP-D in type II alveolar epithelial cells and the regulation of SP-D by hormone. Methods (1)The experiment was divided into COPD group and control group, and according to whether there was a history of smoking addiction, they were further divided into smoking addiction group and non-smoking addiction group. Bronchoscopy and bronchoalveolar lavage were performed in all groups. The expression of SP-D in BALF was detected.(2)Human type II alveolar epithelial cell line was cultured, and they were divided into control group and cigarette smoke extract group. Control group: Cells were cultured in serum-free medium. Cigarette smoke extract group: Cigarette smoke extract was added to the culture medium. After 12 hours, the expression of SP-D was detected, and then dexamethasone was added into the culture medium. After 72 hours, the SP-D level in the culture medium was detected. Results The expression of SP-D in BALF in COPD patients was significantly lower than that in the control group. In the COPD group, SP-D in BALF in the smoking addiction group was significantly lower than that in the non-smoking addiction group. In the control group, SP-D in BALF was significantly lower than that in non-smoking addiction group. In vitro experiments showed that the expression of SP-D in the cell culture medium in the cigarette smoke extract intervention group was significantly lower than that in the control group. The expression of SP-D treated with dexamethasone in the cell culture medium in the cigarette smoke extract intervention group was significantly increased. There was no significant change of SP-D expression in the control group before and after dexamethasone treatment. Conclusion The expression of SP-D in BALF was significantly lower in COPD patients. Smoking addiction could down-regulate the expression of SP-D in BALF. The expression of SP-D in type Ⅱ alveolar epithelial cell culture medium treated with cigarette smoke extract is significantly decreased, and hormone up-regulates SP-D expression in type Ⅱ alveolar epithelial cells.
[Key words] Surfactant protein D; Chronic obstructive pulmonary disease(COPD); Cigarette smoke; Glucocorticoid;Bronchoalveolar lavage fluid
慢性阻塞性肺疾病(chronic obstructive pulmonary disease,COPD)是一种以不完全可逆的气流受限为特征的肺部慢性疾病,气流受限不完全可逆,呈进行性发展,与肺部对有害气体或有害颗粒的异常炎症反应有关,它是呼吸系统的常见病和多发病,现阶段我国约有2500万COPD患者,每年约有100万人死于COPD[1]。因此,鉴于COPD的高发病率,研究影响该疾病发生发展的干预因素,将具有重大的经济效益和社会效益。肺表面活性物质替代治疗在新生儿呼吸窘迫综合征方面已经取得了令人满意的临床效果。近期研究发现,肺表面活性物质D(surfactant protein D,SP-D)在维持小气道稳定、减低气道阻力方面亦起重要作用,并可从多方面参与气道免疫反应的调节[2]。本研究通过研究嗜烟以及不嗜烟的COPD患者和正常人支气管肺泡灌洗液(bronchi alveolar lavage fluid,BALF)中SP-D的表达,研究香烟烟雾提取物对人类Ⅱ型肺泡上皮细胞分泌SP-D的影响以及激素对SP-D表达的调节作用。
1 资料与方法
1.1 一般资料
随机选择2014年6月~2016年6月我院呼吸内科住院及门诊受试患者。COPD组:(1)入组标准:①符合COPD诊断标准;②性别不限;③年龄45~65岁;(2)排除标准:①恶性肿瘤患者;②自身免疫疾病患者;③糖尿病患者;④艾滋病患者;⑤各种精神疾病患者。对照组:肺功能测定FEV1>70%、无肺部及其他系统疾病者。其中COPD组25例,根据有无嗜烟分为嗜烟组(14例)、不嗜烟组(11例)。所有病例均符合世界卫生组织(WHO)制定的COPD诊断标准[3],吸入支气管扩张剂后FEV1/FVC<70%,支气管舒张试验阴性,同时本组患者符合以下指标:Vmax50%<70%预计值,Vmax25%<70%预计值,FEF 25%~75%<70%預计值。所有病例均处于稳定期。对照组17例,根据有无嗜烟分为嗜烟组(10例)、不嗜烟组(7例)。嗜烟组入选标准为:吸烟指数≥300支/年,吸烟指数=平均每天吸烟支数×吸烟年数。
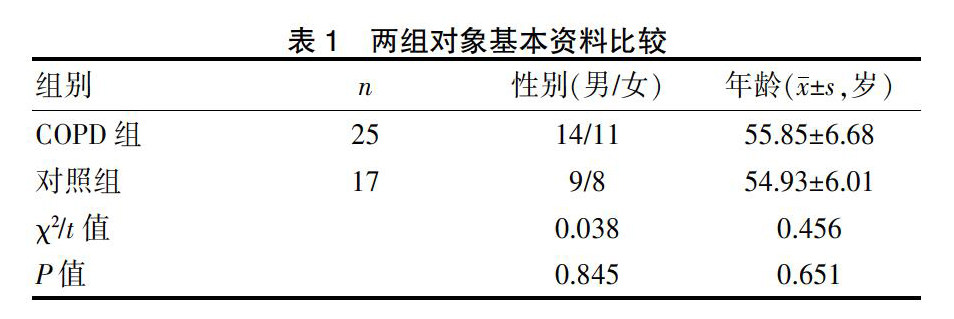
COPD组、对照组患者的性别、年龄比较均无统计学意义(P>0.05),具有可比性。见表1。
实验所用Ⅱ型肺泡上皮细胞来源于A549系,购自中国科学院细胞库(上海),细胞培养于RPMI1640培养液中,置于37℃,5% CO2孵箱中培养。
1.2研究方法
各组患者行常规电子支气管镜检查,并予150 mL生理盐水行支气管肺泡灌洗,回收率大于80%。
制备香烟烟雾提取物[4](cigarette smoke extract,CSE):将2支去过滤嘴香烟于一个注射器驱动装置连接抽吸而燃着,10 min燃完,吸入的烟雾经另一个出口通入50 mL无血清培养液中制成悬液,悬液用1 mol/L NaOH调至pH 7.4,经0.22 μm微孔滤膜过滤备用,制备的香烟烟雾提取物在30 min之内用于实验。各组处理方法:(1)对照组细胞在无血清的RPMI1640培养液中培养。(2)香烟烟雾提取物组细胞RPMI1640培养液中加入香烟烟雾提取物共同培养,模拟香烟对肺泡细胞的刺激作用。各组均在12 h 后留取部分培养液上清,-70℃保存。ELISA法检测SP-D的表达。然后以地塞米松(DEX)2 μmol加入培养液中继续培养细胞。72 h 留取各组细胞培养上清液,-70℃保存待检。
1.3 观察指标
ELISA法检测各组患者支气管肺泡灌洗液以及细胞培养液中 SP-D 的表达水平。采用R&D公司试剂盒,严格按试剂盒说明书进行。
1.4 统计学方法
所有数据均用SPSS 17.0软件进行分析,计量资料以(x±s)表示,同组治疗前后比较采用配对t检验,两组比较采用独立样本t检验,P<0.05表示差异有统计学意义。
2 结果
2.1各组支气管肺泡灌洗液中SP-D的表达
COPD组患者支气管肺泡灌洗液中SP-D表达明显低于对照组,COPD组中嗜烟组支气管肺泡灌洗液中SP-D低于不嗜烟组,差异均有统计学意义。对照组中嗜烟组支气管肺泡灌洗液中SP-D低于不嗜烟组,差异有统计学意义。见表2。
2.2 各组细胞培养液SP-D的表达
与对照组比较,香烟烟雾提取物组细胞培养液中SP-D表达明显降低,差异有统计学意义。香烟烟雾提取物组经地塞米松处理后细胞培养液中SP-D表达水平有明显增加,差异有统计学意义。而对照组经地塞米松处理前后细胞培养液中SP-D表达水平无统计学意义。见表3。
3 讨论
肺表面活性物质是由Ⅱ型肺泡上皮细胞和呼吸性细支气管管壁上的Clara细胞合成和分泌的脂质与蛋白质的复合物,其主要生理功能是降低肺泡表面张力,防止肺萎陷和肺水肿[5-7]。目前发现有4种表面活性物质相关蛋白[8],包括亲水性的SP-A、SP-D和疏水性的SP-B和SP-C,SP-B和SP-C促进磷脂在肺泡表面膜的形成,降低肺泡表面张力,而SP-A和SP-D主要参与调节肺部天然免疫防御作用[9]。肺表面活性物质在生理情况下具有降低肺泡表面张力、抗水肿、稳定小气道、抗氧化和蛋白酶所致肺损伤、调节局部免疫和炎症反应等作用[10-12]。研究显示,应用外源性肺表面活性物质进行干预可改善COPD肺功能,明显抑制气道炎症,减轻肺组织损伤,抑制吸烟及弹性蛋白酶对肺组织的损伤,保护肺泡Ⅱ型上皮细胞的结构和分泌肺表面活性物质的功能,对COPD的发生起到预防和保护作用[13-15]。肺表面活性物质合成分泌障碍参与气道阻塞发病过程,通过适当补充外源性肺表面活性物质或研制可能刺激内源性肺表面活性物质合成分泌的药物,可能会开辟COPD新的治疗途径。
SP-D是肺表面活性相关蛋白的一种,对肺表面活性物质维持正常的生理功能有重要作用,可与各种细菌,病毒和真菌相互作用,使病原体更容易被吞噬细胞吞噬[16]。研究证明,SP-D基因缺陷型老鼠容易产生肺部炎症,引起局部巨噬细胞聚集以及基质金属蛋白酶水平升高,而且更易发生肺气肿和肺损伤,可能与不能有效地清除坏死或凋亡细胞及它们的代谢物质有关[17,18]。体外实验表明,SP-D可与铜绿假单胞菌相互作用促进清除,也可促进对烟曲霉菌孢子的吞噬和杀伤,支气管束中存在的肺表面活性物质可通过减轻气道壁萎陷及防止液桥形成等,在维持小气道稳定、减低气道阻力等方面起重要作用[19,20],可能在COPD发病中有重要作用。
吸烟是慢性阻塞性肺疾病的一个重要的危险因素,在体内和体外的实验均表明香烟的烟雾可以导致巨噬细胞坏死,而细胞坏死可能是肺组织破坏发展成肺气肿的机制之一。有研究证明,COPD患者诱导痰中SP-D的表达可被明显下调。本研究结果表明,COPD患者以及嗜烟者支气管肺泡灌洗液中SP-D表达均明显下降,这与有关学者对诱导痰SP-D的研究结果相一致。同样,我们通过人类Ⅱ型肺泡上皮细胞培养实验表明,在香烟烟雾提取物刺激下Ⅱ型肺泡上皮细胞SP-D的分泌明显抑制,结果同我们的整体实验结果不谋而合。通过地塞米松对细胞培养液中Ⅱ型肺泡细胞的干预表明,使用糖皮质激素对香烟烟雾提取物干预组的Ⅱ型肺泡细胞的SP-D分泌具有明显上调作用,表明糖皮质激素有助于SP-D的合成,糖皮质激素可通过上调SP-D表达这一机制而对COPD具有治疗作用。
[参考文献]
[1]蔡柏蔷.慢性阻塞性肺疾病急性加重诊治中国专家共识(草案).中国呼吸与危重监护杂志,2013,32(6):541-551.
[2]Moreno D,Garcia A,Lema D,et al. Surfactant protein D in chornic obstructive pulmonary disease(COPD)[J]. Recent Pat Endocr Metab Immune Drug Discov,2014,8(1):42-47.
[3]中華医学会呼吸病学分会慢性阻塞性肺疾病学组.慢性阻塞性肺疾病诊治指南(2013年修订版)[J]. 中国医学前沿杂志(电子版),2014,6(2):67-80.
[4]Park JW,Yoon JY,Kim YJ,et al.Extracellular signal-regulated kinase(ERK) inhibition attenuates cigarette smoke extract(CSE) induced-death inducing signaling complex(DISC) formation in human lung fibroblasts(MRC-5)cells[J]. J Toxicol Sci,2010,35(1):33-39.
[5]Wright JR. Immunoregulatory functions of surfactant proteins[J]. Nat Rev Immunol,2005,5(1):58-68.
[6]Orgeig S,Hiemstra PS,Veldhuizen EJ,et al. Recent advances inalveolar biology:Evolution and function of alveolar proteins[J]. Respir Physiol Neurobiol,2010,173(Supp l):S43-S54
[7]Maina JN,West JB,Orgeig S,et al. Recent advances into understanding some aspects of the Structure and function of mammalian and avian lungs[J]. Physiol Biochem Zool,2010,83:792-807
[8]Whitsett JA,Weaver TE. Hydrophobic surfactant proteins in lung function and disease[J]. N Engl J Med,2002,347(26):2141-2148.
[9]Liamina SV,Shimshelashvili ShL,Malyshev IIu. Surfactant protein D-endogenous regulator of inflammation and immune defense[J]. Patol Fiziol Eksp Ter,2012,(1):60-66.
[10]Shanmuga Priyaa Madhukaran,Aghila Rani Koippallil et al.Expression of surfactant proteins SP-A and SP-D in murine decidua and immunomodulatory effects on decidual macrophages[J]. Immunobiology,2016,221(2):377-386.
[11]Gomez-Gil L,Schurch D,Goormaghtigh E,et al. Pulmonary surfactant protein SP-C counteracts the deleterious effects of cholesterol on the activity of surfactant films under physiologically relevant compression-expansion dynamics[J]. Biophys J,2009,97:2736-2745.
[12]Muhlfeld C,Becker L,Bussinger C,et al. Exogenous surfactant in ischemia/reperfusion:Effects on endogenous surfactant pools[J]. J Heart Lung Transplant,2010,29:327-334.
[13]Schleh C,Rothen-Rutishauser BM,Blank F,et al. Surfactant Protein D modulates allergen particle uptake and inflammatory response in a human epithelial airway model[J].Respir Res,2012,1(13):8.
[14]Duvoix A,Miranda E,Perez J,et al. Evaluation of full-length,cleaved and nitrosylated serum surfactant protein D as biomarkers for COPD[J]. Copd-Journal of Chronic Obstructive Pulmonary Disease,2011,8(2):79-95.
[15]Jain D,Atochina-Vasserman EN,Tomer Y,et al. Surfactant protein D protects against acute hyperoxic lung injury[J]. Am J Respir Crit Care Med,2008,178(8):805-813.
[16]Hillaire ML,Haagsman HP,Osterhaus AD,et al. Pulmonary surfactant protein D in first-line innate defence against influenza A virus infections[J]. J Innate Immun,2013,5(3):197-208.
[17]Liu W,Ju CR,Chen RC,et al. Role of serum and induced sputum surfactant protein D in predicting the response to treatment in chronic obstructive pulmonary disease[J]. Exp Ther Med,2014,8(4):1313-1317.
[18]Bersani L,Speer CP,Kunzmann S. Surfactant proteins A and D in pulmonary diseases of preterm infants[J]. Expert Rev Anti Infect Ther, 2012,10(5):573-584.
[19]Gaunsbaek MQ,Rasmussen KJ,Beers MF,et al. Lung surfactant protein D(SP-D)response and regulation during acute and chronic lung injury[J]. Lung,2013,191(3):295-303.
[20]Qi L,Kash JC,Dugan VG,et al. The ability of pandemic influenza virus hemagglutinins to induce lower respiratory pathology is associated with decreased surfactant protein D binding[J]. Virology,2011,412:426-434.